Key takeaways:
~ Acetaminophen can cause liver damage at higher levels or with chronic use, but only in some people.
~ Genetic variants interact with acetaminophen in the risk of liver damage.
~ Understanding your genetic risk, along with lifestyle factors, can help you decide which pain reliever may be the best choice for you.
Members will see their genotype report below, plus additional solutions in the Lifehacks section. Consider joining today.
Acetaminophen, liver damage, and genes:
Click here to listen to this article
Acetaminophen, commonly known by the brand name Tylenol or as paracetamol in Europe, is a widely used over-the-counter medication for pain and fever reduction. While generally safe when taken as directed, higher doses of acetaminophen can lead to severe liver damage and potentially fatal liver failure.
In fact, acetaminophen-related liver damage is currently the most common cause of liver transplant in the US, with more than 56,000 emergency room visits per year from acetaminophen-related liver problems.[ref]
In this article, we’ll explore the research on how liver damage occurs with acetaminophen use and why genetic variants can increase the risk of liver damage at levels that most people can handle.
Metabolism (breakdown) of acetaminophen:
When you take acetaminophen, it is easily and rapidly absorbed in the intestines and then transported to the liver for processing.
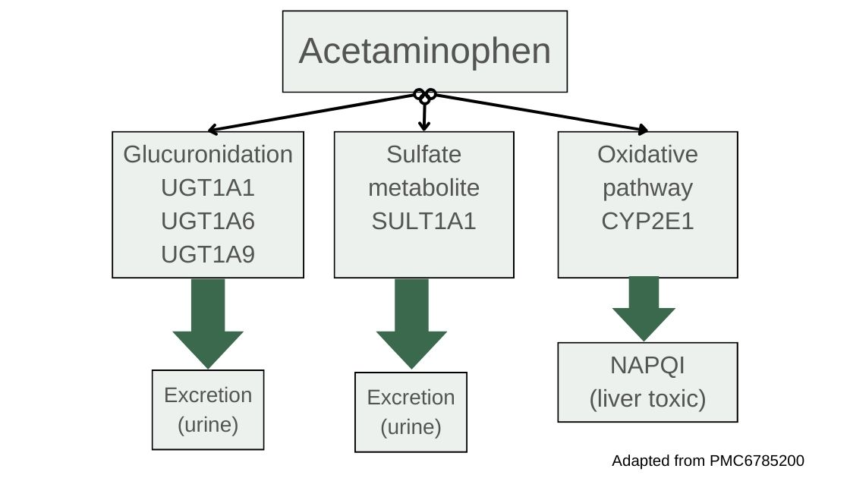
Most of the acetaminophen will be metabolized through conjugation reactions using the SULT and UGT enzymes. The SULT family of enzymes drives conjugation with sulfur groups so that the substance, in this case acetaminophen, can be easily excreted in urine. If the amount of acetaminophen exceeds the capacity of the liver for sulfation, it then utilizes the UGT genes for glucuronidation reactions, which also makes the acetaminophen more water-soluble and able to be excreted. [ref]
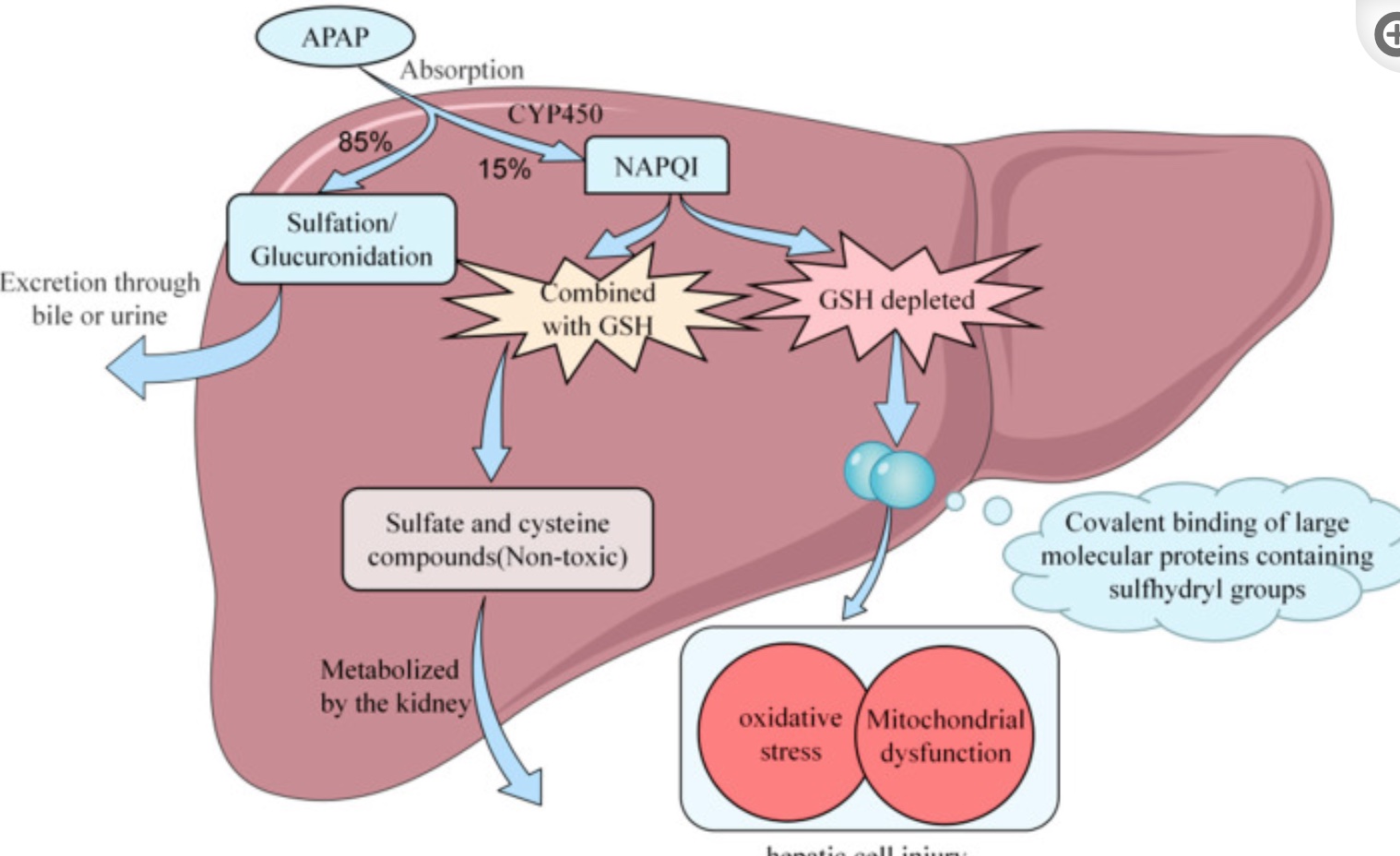
The liver breaks down most of the acetaminophen using sulfation and glucuronidation reactions, allowing for easy excretion. However, about 10-15% of acetaminophen is metabolized by CYP2E1 into a toxic metabolite known as NAPQI (N-acetyl-p-benzoquinone imine).[ref]
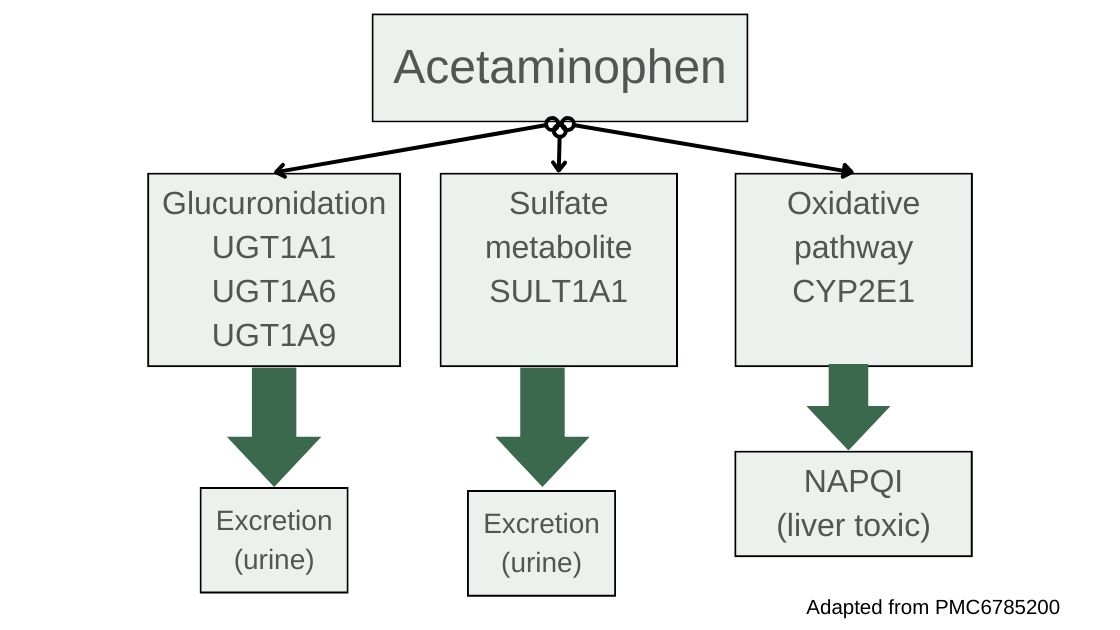
NAPQI then needs to be neutralized with glutathione, which is catalyzed using the GST genes. With sufficient glutathione present, NAPQI is very quickly metabolized into sulfate and cysteine compounds which are readily excreted through the kidneys.[ref] However, when it isn’t neutralized, NAPQI is highly reactive and can cause oxidative stress and damage to the liver cells hepatocytes, leading to cell death.
What goes wrong in acetaminophen-induced liver damage?
When NAPQI levels are higher than the liver’s capacity to neutralize, NAPQI will form protein adducts on the mitochondria of liver cells. This causes high oxidative stress, mitochondrial dysfunction, and cell death of the hepatocytes.[ref] As these cells die, inflammatory cascades perpetuate the oxidative stress, leading to bleeding, fibrosis, or possibly liver failure.
Importantly, people who already have liver disease are at a higher risk of liver damage from acetaminophen because they likely aren’t producing enough glutathione. If you have pre-existing liver damage, talk with your doctor about whether acetaminophen is safe for you.
There are a couple of other scenarios in which acetaminophen is more likely to cause liver damage:
Alcohol Use Disorder:
Normally, alcohol is metabolized using the alcohol dehydrogenase enzymes, but in cases of chronic overconsumption or binge drinking, the CYP2E1 enzyme is upregulated to metabolize the extra alcohol. People who drink a lot on a regular basis are more likely to have the CYP2E1 enzyme constantly upregulated, potentially increasing the conversion of acetaminophen into the toxic metabolite, NAPQI. [ref]
Glutathione System Overwhelmed:
Under normal circumstances, NAPQI is quickly neutralized by conjugation with glutathione, a powerful antioxidant found in liver cells, and then excreted from the body. However, when excessive amounts of acetaminophen are ingested, the liver’s supply of glutathione can be depleted rapidly, leaving NAPQI unneutralized.[ref]
In infants and in utero:
In young babies, the sulfation and glucuronidation pathways are not fully formed, and the metabolism of acetaminophen is shifted in utero and in preterm babies potentially increasing the risk of problems.[ref][ref]
Genetic interactions with acetaminophen:
With multiple routes of detoxification, most people can take acetaminophen without liver damage. Even if one gene is slightly impaired, the other detoxification pathways can compensate. However, a small percentage of the population may still encounter problems from chronic acetaminophen usage or high doses.
Genetic studies on the topic are actually pretty extensive, and several genetic variants have been identified that increase the risk of liver damage from chronic or high acetaminophen usage.
For example, more than a decade ago, researchers found that the CD44 gene was likely involved in the risk of liver damage from high doses of acetaminophen. For the chronic dosing studies, most studies are looking at doses of 4 g/day.[ref] (This used to be the maximum recommended acetaminophen dose per day, but that now has changed to 3g/day.) For reference, a single over-the-counter Extra Strength Tylenol is 500 mg, with the directions saying not to exceed 6 tablets in 24 hours (so 3g/day). Prescription doses can be up to 4g/day.[ref]
With the research pointing to CD44 and increased liver damage markers, researchers used mouse models and human genetics to understand which SNP (single nucleotide polymorphism) causes the increased risk.[ref][ref]
What is CD44? The CD44 gene encodes a cell-surface receptor involved in various processes, including cellular differentiation, cell-to-cell adhesion, recruitment of immune system cells, and as a receptor for hyaluronic acid. Studies in humans and animals with non-alcoholic fatty liver disease show that CD44 plays a key role in the inflammation and liver damage in NAFLD.[ref]
Other genetic studies have looked at genes in pathways known to be involved in acetaminophen metabolism, including genes related to sulfation, glucuronidation, and glutathione production.
For example, a study that looked at genetic differences in a group of 192 individuals who took 4 g/day of acetaminophen found that variants that decreased sulfation (specifically in the SULT1E1 gene) were more likely to have elevated liver enzymes. The study showed a huge variation in liver enzyme levels (image below), but it didn’t identify any specific SNP. It just showed that SULT1E1 was likely involved and that combinations of SULT variants plus GST variants were likely deleterious.[ref]
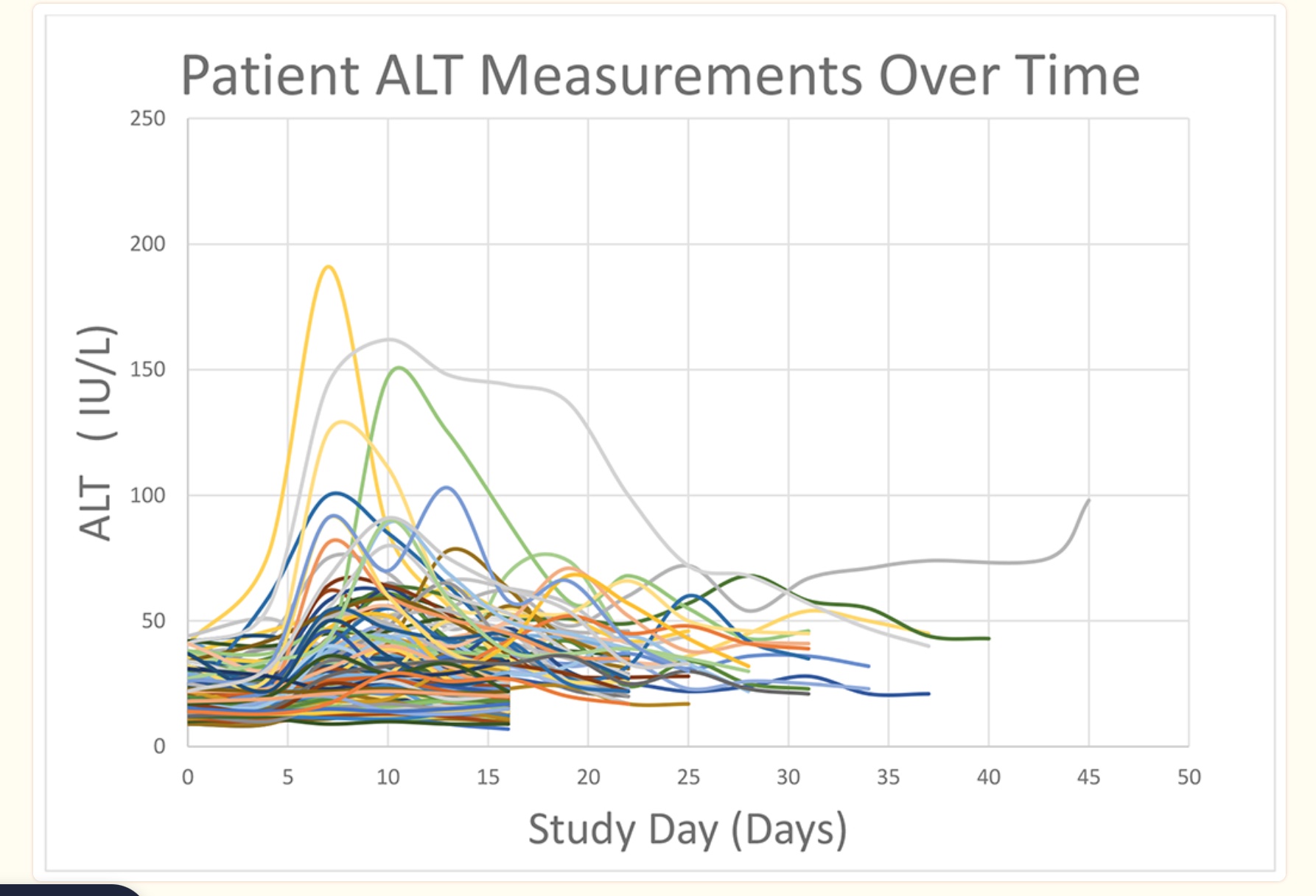
Another cell line genome-wide study found more than 22 genes related to susceptibility to acetaminophen-induced liver damage. The combination of variants linked to a slightly increased risk of liver damage could add together to increase risk more significantly. [ref]
In summary, knowing your risk factors may help you figure out whether acetaminophen is a safe choice for you as a pain reliever at higher doses or for longer periods of time. The genotypes section below includes genetic variants associated with liver problems from acetaminophen, but please keep in mind that more research is needed on this topic to truly understand individual risk.
I have to admit that I’m wondering why there isn’t more definitive research on this topic for an OTC drug that is the number one cause of liver transplants…
Genotype report: Acetaminophen and liver damage
The genotypes section of the article includes genetic variants associated with liver problems from acetaminophen, but if you have any concerns about your acetaminophen use, it’s always best to consult with your healthcare provider.
Member Content:
Why join Genetic Lifehacks?
~ Membership supports Genetic Lifehack’s goal of explaining the latest health and genetics research.
~ It gives you access to the full article, including the Genotype and Lifehacks sections.
~ You’ll see your genetic data in the articles and reports.
Join Here
Related articles and topics:
About the Author:
Debbie Moon is the founder of Genetic Lifehacks. Fascinated by the connections between genes, diet, and health, her goal is to help you understand how to apply genetics to your diet and lifestyle decisions. Debbie has a BS in engineering from Colorado School of Mines and an MSc in biological sciences from Clemson University. Debbie combines an engineering mindset with a biological systems approach to help you understand how genetic differences impact your optimal health.