Key takeaways:
~ Manganese is an essential trace mineral that is needed in the right amounts. Too much or too little is detrimental to health.
~ Cellular mechanisms regulate the amount of manganese in the body, with much of what you get from food being excreted.
~ Genetic variants in manganese transporter genes can affect how much you get from your diet.
Members will see their genotype report below and the solutions in the Lifehacks section. Consider joining today.
Why is manganese important? How do your genes affect your need for manganese?
Manganese is a trace mineral (symbol Mn) that is critical in the right amounts – too much or too little can cause problems.
The body contains 10 to 20 mg of manganese, with up to 40% of it being found in the bones. The recommended adequate intake for manganese is 1.8 mg/day for adult women (slightly more if pregnant or breastfeeding) and 2.3 mg/day for men.[ref]
While frank manganese deficiency is rare in humans, under certain circumstances, manganese deficiency can cause skeletal abnormalities, impaired glucose tolerance, hair depigmentation, and slowed growth in children. [ref][ref][ref]
- A study involving healthy young men found that a manganese-depletion diet for 39 days caused a decrease in cholesterol and most developed a transient skin rash.
- In another study of healthy young women, two months of either consuming either 0.7 mg Mn/day or 9.5 mg Mn/day did not affect their manganese status, but it did affect their iron levels (more on iron and manganese below).
- Another study in women found that low Mn intake increased symptoms during their menstrual cycle.
Manganese toxicity can occur with excessive exposure to this metal, especially inhalation in industrial settings. Excess manganese can accumulate in the bones, kidneys, liver, pancreas, and especially in the brain. Symptoms of manganese overload can include Parkinson’s-like symptoms, liver problems, and bone health issues. Excess manganese causes oxidative stress by interfering with mitochondrial energy production. In the brain, manganese is preferentially stored in dopaminergic neurons, which is why it causes Parkinson’s symptoms.[ref][ref]
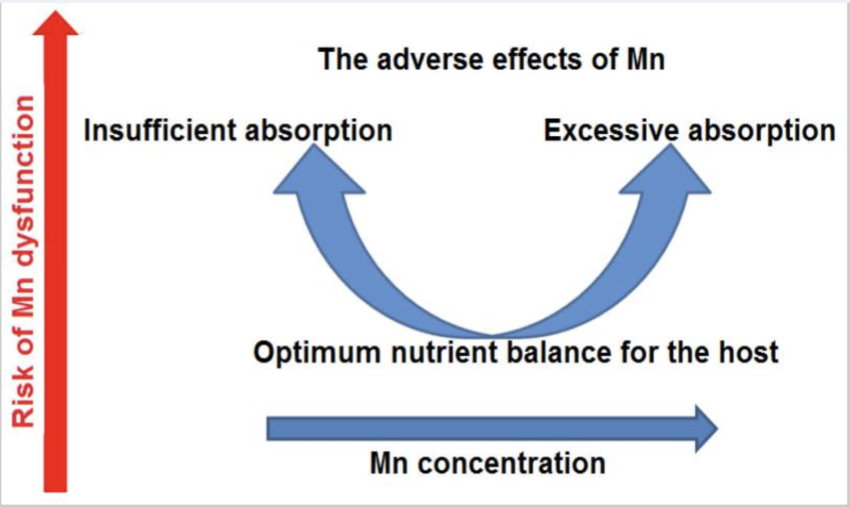
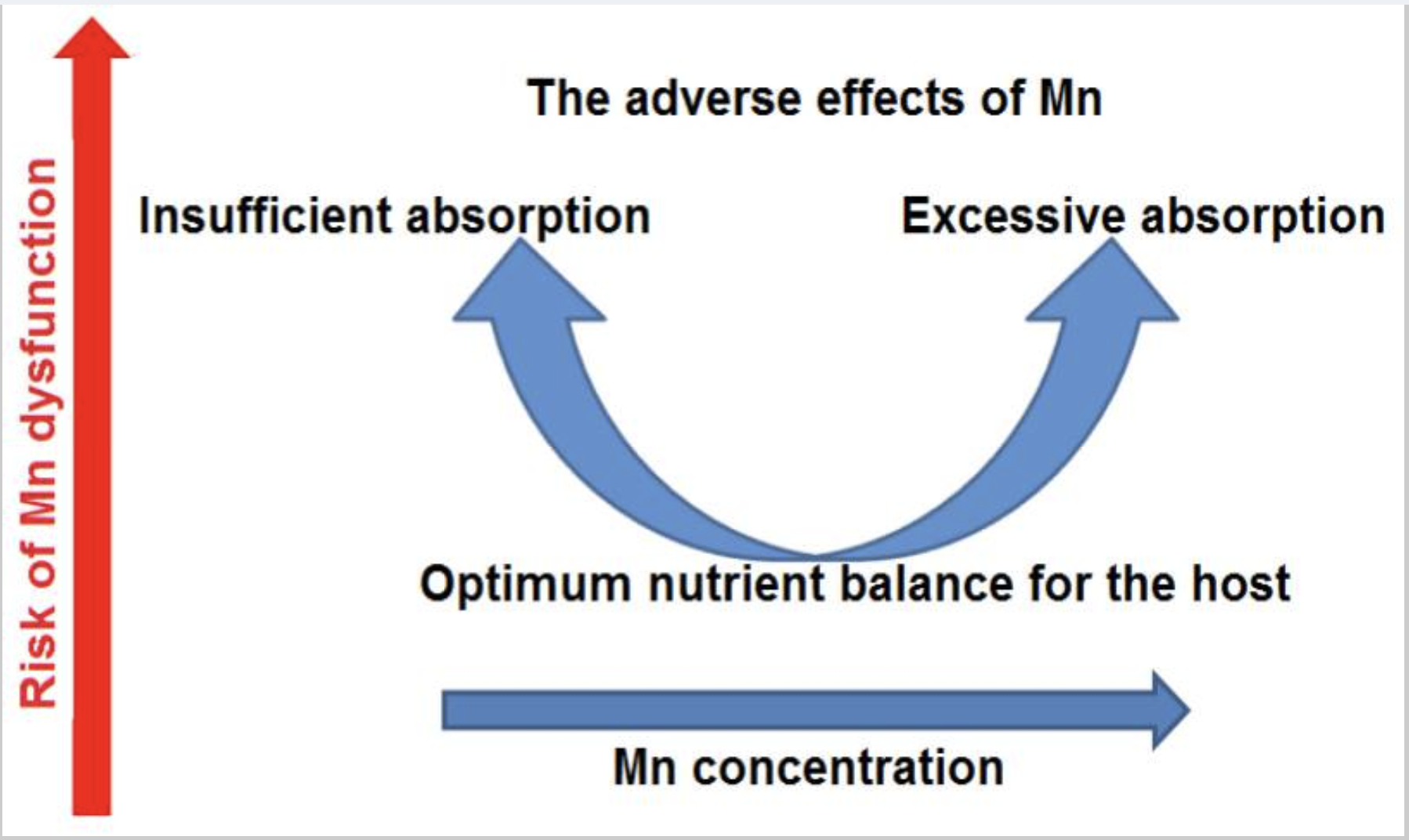
Balance is key with manganese.
Higher manganese levels are associated with symptoms such as: [ref][ref]
- Behavioral disorders in children
- Unstable emotional behavior
- Neurodegenerative disorders
On the other hand, low manganese levels can increase the risk of osteoporosis and cause slow wound healing.
Roles of Managenase in the Body:
Manganese is an essential trace element — meaning that it is needed for vital functions but at very low levels. The body tightly regulates how much manganese is available, with much of what is absorbed in the intestines being excreted in the bile.
Let’s take a look at some of manganese’s roles, illustrating both why it’s essential and why excess can be a problem.
Antioxidant functions:
Manganese superoxide dismutase (MnSOD) is an enzyme produced in cells that acts as an antioxidant. In short, cells produce reactive oxygen species (ROS) when they make ATP for energy. The amount of ROS in cells must be kept low to prevent oxidative stress, and MnSOD helps regulate ROS levels. Specifically, MnSOD converts superoxide anion radicals, which are the major source of cellular ROS, to hydrogen peroxide and oxygen in the mitochondria.[ref]
MnSOD uses manganese as a cofactor, which is one big reason why having enough manganese available is essential for wellness. However, the MnSOD enzyme can also bind to iron instead of manganese, generating reactive hydroxyl radicals (pro-oxidant) in some tissues or situations. For example, in certain types of breast cancer, MnSOD acts as a pro-oxidant.[ref]
MnSOD is essential for combating oxidative stress, and low levels of MnSOD are found in chronic diseases, such as diabetes, cardiac fibrosis, and neurodegenerative diseases.[ref]
Bone, cartilage, and collagen:
Glycosylation is a common modification of proteins in which a carbohydrate is attached to the molecule, and manganese is used in some glycosylation reactions. One such reaction is the synthesis of proteoglycans, which are used to produce cartilage and bone. Additionally, manganese is a cofactor for an enzyme used in collagen synthesis, which makes manganese important for wound healing.[ref]
In rare situations with very low levels of manganese due to mutations, the lack of glycosylation can cause severe problems including a deformed skull, short limbs, and seizures.[ref]
People with osteoporosis have lower manganese, copper, and zinc in the cancellous bones.[ref] However, it isn’t clear whether low manganese causes osteoporosis or if it is the lack of another mineral (e.g. zinc or copper) driving the changes since they use the same transporters.
Amino acid metabolism:
Manganese is a cofactor for both the arginase and glutamine synthetase enzymes.[ref]
- Arginase: In the urea cycle, arginase hydrolyzes arginine to produce urea and ornithine. This reaction is essential for detoxifying ammonia, a byproduct of amino acid metabolism, into urea for excretion. Arginase is also key in regulating the concentration of arginine and ornithine, which can then affect glutamine, glutamate, and inducible nitric oxide. In addition, arginine levels are important for wound healing. [ref]
- Glutamine Synthetase: This enzyme catalyzes the ATP-dependent conversion of glutamate and ammonia to form glutamine. Glutamine is an important amino acid. It is part of protein synthesis and purine synthesis, and it can also be used by cells for energy production (conversion to alpha-ketoglutarate).
Note that both arginase and glutamine synthetase can affect glutamine and glutamate levels. Carriers of mutations that cause extremely low levels of manganese have a higher risk of schizophrenia, which is also linked to glutamate regulation in the brain and oxidative stress.[ref]
Related articles: Glutamate synthesis and transport and Schizophrenia genes
Interaction with CoQ10:
Excess manganese in cells can cause the degradation of CoQ7, which is essential in the biosynthesis of CoQ10. The study was done using yeast as a model, but the mechanism should be the same in humans. Essentially, excess manganese interferes with mitochondrial CoQ10 production and disrupts mitochondrial energy production at complex III.[ref]
Balancing Manganese Levels in the Body:
Since we need just the right amount of manganese, the body has multiple ways that it can balance it out. Essentially, the intestines regulate how much is absorbed. The liver clears out any excess manganese that is circulating in the blood by secreting it in bile for fecal excretion. Cells can take in manganese with specific transporters, and they can export excess manganese back out of the cell with other transporters.[ref]
Let’s dive into the details here, because this is where genetic variants come into play.
Absorption of Manganese and Interaction with Iron:
In a healthy adult, only about 1-5% of ingested manganese is absorbed in the intestines (around 1% for men, 4% for women) and the rest is excreted immediately. Men tend to absorb less manganese due to higher average iron levels.
Higher dietary levels of manganese cause an adaptive change that reduces intestinal absorption. Manganese can also be absorbed by the lungs, usually from industrial exposure.[ref]
Let’s take a look at how manganese is absorbed and regulated.
Manganese can’t just flow into a cell, including in the intestines at dietary levels. It’s taken up by receptors that bind to and transport it across the cell membrane.
Iron and manganese are both divalent metals and use the same uptake transporters in the intestine and other tissues. Intestinal transporters have a higher affinity for iron and are more focused on iron homeostasis. Thus, dietary iron deficiency can upregulate the transporters and lead to increased absorption of manganese (also lead and cadmium).
The intestinal cell receptors used to transport iron and manganese include the transferrin receptor (Tf), divalent metal transporter 1 (DMT1), lactoferrin receptor, and ferroportin. [ref][ref]
Almost all iron circulating in the body is bound to transferrin, and manganese, in the Mn(3+) form, can also circulate and be bound to transferrin. When manganese is in the Mn(2+) form, it is transported into cells by the divalent metal transporter 1 (DMT1).[ref]
The availability of transporters for absorption in the intestines is one of the main ways that both iron and manganese levels are regulated systemically.
Transporter availability: Animal studies show that excess manganese (dietary or supplemental) can impair iron absorption and lead to iron deficiency. The reverse is also true – iron supplementation can reduce blood manganese levels.[ref][ref] The expression of the DMT1 transporter is regulated by the amount of iron already in the body. More iron = less DMT1 transporters in the intestines.[ref]
Studies in mice with DMT1 variants show that both manganese and iron are taken up by the DMT1 transporter in the intestine and nose. The mice with DMT1 transporter mutations had low levels of both iron and manganese. In mice with normal DMT1 genes, iron deficiency (and thus higher DMT1) caused increased manganese absorption.[ref]
What about people with HFE (hemochromatosis) mutations?
The HFE gene encodes codes for a protein that controls how iron is taken into the intestinal cells. As I mentioned above, free iron is bound to transferrin, which binds to a transferrin receptor to be taken into the cell. HFE can also bind to the transferrin receptor, blocking the receptor from taking iron into the cells. In this way, HFE is a negative regulator of iron absorption.[ref]
There are two good human studies that show the interaction between HFE mutations and manganese:
- A Harvard study showed that variants in the HFE gene that can cause hemochromatosis are associated with high iron absorption and lower manganese levels in pregnant women.[ref]
- A 2024 genome-wide association study identified the HFE C282Y variant as being associated with lower serum manganese levels.[ref]
Studies in mice with an HFE mutation show that manganese levels are also lower when manganese is inhaled, as is the case in industrial settings. Importantly (for someone with an HFE variant), animal studies also show that there is a decrease in iron transporters, including DMT1, for moving iron and manganese into cells including in the brain.[ref] Another study in mice showed that excess manganese exposure in normal mice causes impulsivity and neurotoxicity, but in HFE mutant mice, the negative effects of manganese exposure in the brain aren’t seen.[ref]
However, not all studies show a clear association of lower manganese with the HFE mutations. An older mouse study on HFE showed that manganese absorption increased in the intestines. Plus, a study looking primarily at manganese in prion disease also showed that blood manganese levels were higher in people with hemochromatosis (small study, n=19 with hemochromatosis compared to a small control group).[ref] (I’m not sure if there are other factors influencing manganese in hemochromatosis patients included in the prion study.)
In addition to hemochromatosis, other blood disorders are also associated with altered manganese levels. Beta thalassemia major is an inherited blood disorder that causes reduced hemoglobin production. Studies show that people with beta thalassemia are likely to have higher levels of both iron and manganese.[ref]
Excretion of Manganese:
The body maintains the right manganese levels by both limiting absorption and maximizing excretion.
Approximately 1-5% of dietary manganese is absorbed in the intestines, as discussed above, and then travels to the liver before circulating in the bloodstream.
In a healthy liver, more than 90% of the manganese absorbed from your diet is excreted back out via bile. A study using radiolabeled manganese showed that the retention of manganese 10 days after ingestion was about 5% in women.[ref] In people with liver dysfunction, more manganese can be retained in the body, which leads to neurotoxicity. One study showed that a liver transplant in patients with cirrhosis normalized brain manganese levels within three months.[ref]
Genes Related to Cellular Manganese Levels
We’ve covered the big picture of how manganese is absorbed and how it is excreted back out via bile. Let’s move on to how manganese levels are maintained in cells because that is where the problems with deficiency or with excess happen.
Manganese homeostasis is tightly regulated, and several genes are involved in its transport and regulation at the cellular level.
SLC30A10 (aka ZNT10):
This gene encodes a protein that functions as a manganese efflux transporter (moves Mn out of the cells for excretion). Rare mutations in SLC30A10 can lead to manganese accumulation and toxicity, resulting in conditions such as familial manganese-induced parkinsonism. A more common variant (below in the genotype report) can increase manganese levels in the body.
SLC39A8 (aka ZIP8):
This gene encodes a manganese importer and regulator of brain manganese levels. Mutations in this gene can result in manganese deficiency and are associated with developmental delay, intellectual disability, and other health issues due to lack of MnSOD activity.[ref]
Mutations that decrease SLC39A8 are linked to a Congenital Disorder of Glycosylation (CDG) “due to the exquisite sensitivity of glycosyltransferases to Mn concentration.”[ref]
SLC39A14 (aka ZIP14):
Encodes a primary Mn transporter that is important in regulating the brain uptake of manganese and preventing neurotoxicity. It is also essential in the way that liver cells take up manganese for excretion via bile.[ref] Mutations in SLC39A14 impair Mn uptake, without affecting iron, zinc, or cadmium. Rare mutations cause childhood-onset parkinsonism-dystonia. [ref] Note that there are no SLC39A14 common variants in the genotype report – this is an essential gene and rare changes to it cause significant problems.
ATP13A2:
This gene encodes a lysosomal P-type ATPase involved in the homeostasis of manganese and other divalent metals. Rare mutations in ATP13A2 are linked to a form of juvenile-onset parkinsonism known as Kufor-Rakeb syndrome due to a lack of manganese.[ref]
Next up in the Genotype report section, you’ll see how your genes affect these manganese transporters.
Genotype report: Manganese
Lifehacks:
We have about 10 to 20 mg of manganese in our bodies, up to 40% of which is stored in our bones. Most of the manganese (90%+) absorbed from your diet is excreted in the bile.[ref]
What can you do with the genetic information?
People with the above genetic variants that increase manganese levels may want to carefully consider how much manganese they consume or are exposed to.
On the other hand, people with variants linked to lower manganese levels may want to increase their dietary manganese intake if it is low. Keep in mind that getting enough manganese is important for antioxidant production.
How can you know how much manganese you get each day?
Cronometer.com is a free web application for tracking what you eat each day. It breaks down your food consumption into both macro and micronutrients – including manganese. Track what you eat for a few days and see how much manganese you get on average. Then take into consideration whether you genetically may need a little more – or a little less – manganese.
Here’s how much manganese is recommended by age group and sex:
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 6 months* | 0.003 mg | 0.003 mg | ||
| 7–12 months | 0.6 mg | 0.6 mg | ||
| 1–3 years | 1.2 mg | 1.2 mg | ||
| 4–8 years | 1.5 mg | 1.5 mg | ||
| 9–13 years | 1.9 mg | 1.6 mg | ||
| 14–18 years | 2.2 mg | 1.6 mg | 2.0 mg | 2.6 mg |
| 19–50 years | 2.3 mg | 1.8 mg | 2.0 mg | 2.6 mg |
| 51+ years | 2.3 mg | 1.8 mg |
Food sources of manganese:
Most people meet their need for manganese through foods, although water can also contain low levels of manganese. Good sources of manganese include nuts, whole grains, rice, legumes, spinach, chocolate, and tea.[ref]
| Food | Milligrams (mg) per serving |
Percent DV* |
|---|---|---|
| Mussels, blue, cooked, 3 ounces | 5.8 | 252 |
| Hazelnuts, dry roasted, 1 ounce | 1.6 | 70 |
| Pecans, dry roasted, 1 ounce | 1.1 | 48 |
| Brown rice, medium grain, cooked, ½ cup | 1.1 | 48 |
| Oysters, Pacific, cooked, 3 ounces | 1.0 | 43 |
| Clams, cooked, 3 ounces 0.9 | 0.9 | 39 |
| Chickpeas, cooked, ½ cup | 0.9 | 39 |
| Spinach, boiled, ½ cup | 0.8 | 35 |
| Pineapple, raw, chunks, ½ cup | 0.8 | 35 |
| Soybeans, boiled, ½ cup | 0.7 | 30 |
| Bread, whole wheat, 1 slice | 0.7 | 30 |
| Oatmeal, cooked, ½ cup | 0.7 | 30 |
| Peanuts, oil-roasted, 1 ounce | 0.5 | 22 |
| Tea, black, brewed, 1 cup | 0.5 | 22 |
| Lentils, cooked, ½ cup | 0.5 | 22 |
| Potato, flesh and skin, baked, 1 medium | 0.3 | 13 |
| White rice, long grain, cooked, ½ cup | 0.3 | 13 |
As you can see, a couple of slices of bread along with a glass of tea and a handful of nuts can meet your daily RDI.
Causes of Excess Manganese Exposure:
The biggest source of manganese overload is inhalation in industrial settings, such as working at a ferromanganese plant or living next to one (air pollution, water pollution).
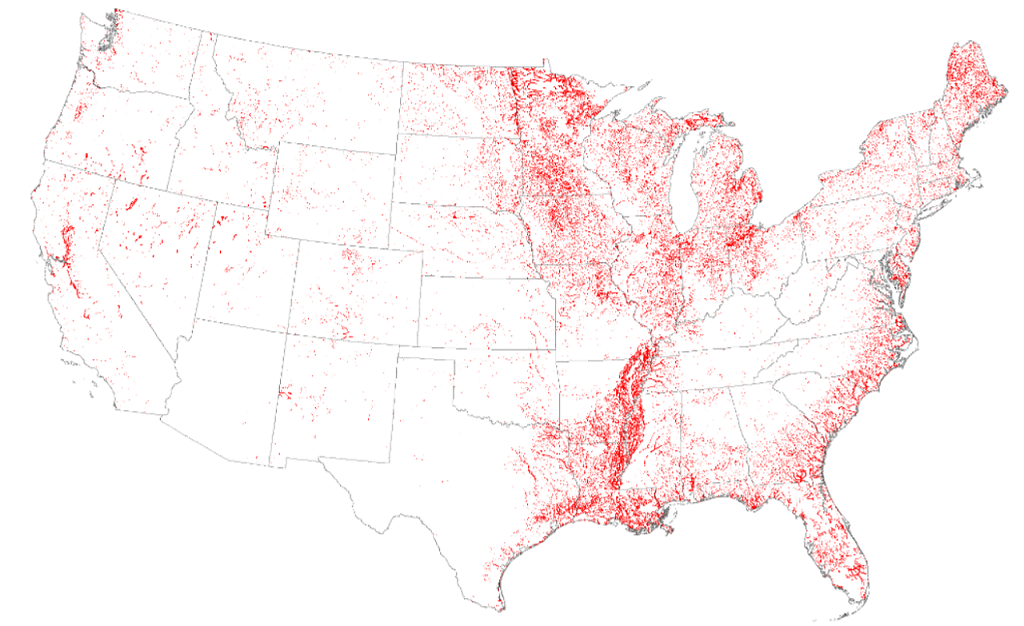
Well Water: Signs of manganese in your water include black stains on your toilet, shower, or laundry.[ref] Even long-term inhalation of Mn-containing water in the shower can potentially pose a risk of neurotoxicity, especially when combined with impaired liver function[ref]
According to the USGS, about 7% of wells have concentrations of manganese at levels that are potentially harmful. Water testing for manganese is readily available and may be a good idea if you use well water in an area known for high Mn levels.
Supplemental Manganese
If you have any questions about whether supplemental manganese is right for you, please talk with your healthcare provider.
In supplements, manganese is usually found in chelated forms such as manganese bis-glycinate chelate, manganese glycinate chelate, and manganese aspartate. It is usually listed on the label as the amount of elemental manganese in the product, and supplements can contain anywhere from 5 to 34 mg of manganese.
Precautions with supplemental manganese:[ref]
- Use caution with supplemental manganese if you have the genetic variant above that increases manganese levels.
- Clinicians and studies caution against using manganese supplements (or high exposure) if you have impaired liver or impaired bile function.
- Children need a lot less manganese than adults because they have higher intestinal absorption of manganese.
- If you are iron deficient, you are likely to absorb more manganese and could have accumulation in the brain.
Should you alter your manganese intake if you have HFE (hemochromatosis) variants?
There’s a popular article by Chris Masterjohn (Iron Overload: Forget what you think you knew) that makes the claim that people with HFE variants should “restrict dietary manganese to no more than two milligrams per day” and 1 mg/day for four weeks after blood donation. He theorizes that people with HFE variants absorb more iron and more manganese based on older studies in rats. Then he goes on to strongly recommend continually restricting manganese as a way to prevent problems with iron overload.
I don’t think the overall research on iron, the HFE variants, and manganese fits with his claims in the article — specifically that manganese overload occurs with iron overload and is the cause of the symptoms and liver dysfunction.
A 2024 genome-wide association study identified the HFE C282Y variant as being associated with lower serum manganese levels. Genome-wide association studies look at the entire genome to see if there is an association with a trait, disease, or biomarker (such as manganese levels). In this case, the HFE C282Y variant was one of seven newly identified genetic markers associated with Mn levels.[ref]
You can decide for yourself… If you have read his article, go ahead and read the rat studies that he chose as the basis for his assertions. Then read the human studies that I included above showing lower manganese with HFE variants[ref][ref], as well as the ones showing that the iron increase with HFE variants seems to downregulate DMT1 and decrease manganese.
Note that I’m not suggesting manganese supplements are needed by people with HFE variants, but rather that manganese from foods doesn’t need to be restricted.
Note that the studies I have referenced for lower Mn levels include people with HFE variants but not necessarily with hemochromatosis. Hemochromatosis is a chronic end-stage disease that can eventually develop due to iron overload and can lead to cirrhosis of the liver. Since more than 90% of dietary manganese is excreted by the liver in the bile, manganese excretion is likely to be impaired in people with liver dysfunction. If you have cirrhosis from iron overload, the liver dysfunction is likely to interfere with manganese excretion…
Most importantly, talk with your doctor about manganese consumption if you have any questions.
Liver Health and Manganese:
Severe liver dysfunction, such as cirrhosis or liver failure, can dramatically reduce the excretion of manganese, leading to excess manganese in the brain and neurodegenerative disorders.
But what about NAFLD (fatty liver disease), which affects liver function somewhat? Two recent studies showed that people with higher blood manganese levels were also more likely to have liver steatosis.[ref][ref] Another study showed that in women, the risk of NAFLD compared to manganese levels was a U-shaped curve. This makes sense when you consider that manganese is needed for MnSOD as an antioxidant, so low manganese could increase oxidative stress in the liver. Similarly, too high of manganese could also either cause or be a result of NAFLD.[ref]
The question with association studies is the direction of causality. Does NAFLD decrease the excretion of manganese in the bile, leading to higher Mn levels in the blood? Or does high manganese exposure cause oxidative stress in the liver, leading to NAFLD?
One study in China found that in areas of high manganese exposure, there was an increased risk of NAFLD. This implies that high manganese intake could cause liver stress and fatty liver.[ref] However, other studies suggest that higher manganese intake through food could help prevent NAFLD through the anti-oxidant effects of MnSOD.[ref]
Related article: NAFLD, Genetics, and Prevention of Fatty Liver
Related articles and topics:
About the Author:
Debbie Moon is the founder of Genetic Lifehacks. Fascinated by the connections between genes, diet, and health, her goal is to help you understand how to apply genetics to your diet and lifestyle decisions. Debbie has a BS in engineering from Colorado School of Mines and an MSc in biological sciences from Clemson University. Debbie combines an engineering mindset with a biological systems approach to help you understand how genetic differences impact your optimal health.